Bone and joint health markers in persons with hemophilia A treated with emicizumab in the HAVEN 3 clinical trial
A systematic review of mortality statistics and causes of death in people with congenital hemophilia A (PwcHA)
Final Results of PUPs B-LONG Study: Evaluating Safety and Efficacy of rFIXFc in Previously Untreated Patients With Hemophilia B
Final Results of PUPs A-LONG Study: Evaluating Safety and Efficacy of rFVIIIFc in Previously Untreated Patients With Hemophilia A
Objective:
PUPs A-LONG aimed to evaluate the safety, including inhibitor development, and efficacy of extended half-life (EHL) recombinant factor VIII Fc fusion protein (rFVIIIFc) in previously untreated patients (PUPs) with severe hemophilia A.
Methods:
This open-label, multicenter, Phase 3 study (NCT02234323) enrolled male PUPs aged <6 years with severe hemophilia A (<1 IU/dL endogenous FVIII) to receive rFVIIIFc. Primary endpoint was inhibitor development (incidence rate=number of patients with inhibitors/number of patients reaching ≥10 exposure days [ED] milestone or with inhibitors). A secondary endpoint was annualized bleed rate.
Summary:
Of 103 patients receiving ≥1 dose, 80 (77.7%) were <1 year old, 20 (19.4%) had a family history of inhibitors, and 82 (79.6%) had a high-risk hemophilia genotype. Eighty-one patients started on episodic treatment; of these, 69 switched to prophylaxis. Twenty patients started on prophylaxis, and 2 were not assigned a regimen. Eighty-seven (84.5%) patients completed the study. Eighty-seven (84.5%), 85 (82.5%), and 81 (78.6%) patients had ≥10, ≥20, and ≥50 EDs to rFVIIIFc, respectively. Total and high-titer (≥5.00 BU/mL) inhibitor rate was 31.1% (28/90) and 15.6% (14/90), respectively, for patients with ≥10 EDs (3 patients with inhibitors and <10 EDs included). Median time to inhibitor development was 9 EDs (range: 1–53). rFVIIIFc dosing and efficacy data are in Table 1. Twenty-eight (27.2%) patients had 32 rFVIIIFc adverse events assessed as related by the investigator (FVIII inhibition, n=28; soft tissue hemorrhage, n=1; deep vein thrombosis, n=1; device-related thrombosis, n=1; papular rash, n=1). There was 1 non–treatment-related death due to intracranial hemorrhage (onset during screening period before first rFVIIIFc dose).
Conclusions:
This was the first prospective study of an EHL, rFVIIIFc, as treatment for PUPs with severe hemophilia A. Overall inhibitor development was within the expected range, although high-titer incidence was lower than that reported in the literature. The data demonstrate that rFVIIIFc was well tolerated and effective in this pediatric patient population.
Four-year safety and efficacy of N8-GP (ESPEROCT®) in previously treated adolescents/adults with hemophilia A in the completed pathfinder 2 trial
Five-year safety and efficacy of N9-GP (REBINYN®) in previously treated children with hemophilia B in the ongoing paradigm 5 trial
Modeling of Daily Administration of N8-GP (ESPEROCT®) vs Standard Half-life FVIII for Patients With Hemophilia A Participating in Sports Activities
Five-year safety and efficacy of N8-GP (ESPEROCT®) in previously treated children with hemophilia A in the completed pathfinder 5 trial
Three-year efficacy and safety results from a phase 1/2 clinical study of AAV5-hFVIII-SQ gene therapy (valoctocogene roxaparvovec) for severe hemophilia A (BMN 270-201 study)
Baseline patient characteristics in ReITIrate: A prospective study of rescue ITI with recombinant factor VIII Fc fusion protein (rFVIIIFc) in patients who have failed previous ITI attempts
Clinical Study to Investigate the Efficacy and Safety of Wilate During Prophylaxis in Previously Treated Patients With Von Willebrand Disease (VWD)
No evidence of germline transmission of vector DNA following intravenous administration of AAV5-hFIX to male mice
Bleeding types and treatments in patients with von Willebrand disease before and after diagnosis
Congenital afibrinogenemia: a case report of perioperative hematological management during difficult orthopedic surgery
Head-to-head pharmacokinetic comparisons of N9-GP with standard FIX and rFIXFc in patients with hemophilia B
Long-term clinical outcomes of rFIXFc prophylaxis in adults 50 years of age or older with severe hemophilia B
Long-term clinical outcomes of rFVIIIFc prophylaxis in adults 50 years of age or older with severe hemophilia A
Efficacy of on-demand treatment of bleeding episodes in hemophilia B patients with extended half-life N9-GP in pivotal trials: an in-depth analysis of treatment
An update on cognitive and behavior function in children and young adults with hemophilia: a 25-year journey from the Hemophilia Growth and Development Study to the current eTHINK study
PROTECT VIII Extension Trial Interim Data: Safety of >5 Years of Treatment With BAY 94-9027
Effective Long-term Prophylaxis with BAY 94-9027 in Previously Treated Children: Interim Results of the PROTECT VIII Kids Extension Study
Long-term Benefit of BAY 81-8973 Prophylaxis in Children With Severe Hemophilia A: Interim Analysis of the LEOPOLD Kids Extension Study
Effective Protection for >5 Years With BAY 94-9027 Prophylaxis: PROTECT VIII Extension Trial Interim Results
BAY 94-9027 Maintains Hemostasis During Major Surgery in Adults and Adolescents With Severe Hemophilia A: PROTECT VIII Results
Objective:
BAY 94-9027 is an extended–half-life recombinant factor VIII (FVIII) product. Efficacy in maintaining hemostasis during major surgery was evaluated in a subset of patients with severe hemophilia A in the phase 2/3 PROTECT VIII study.
Methods:
Patients aged 12–65 years requiring major surgery during PROTECT VIII or its ongoing extension were included in the analysis. Patients with severe hemophilia A undergoing major surgery who were not enrolled in PROTECT VIII but met all study inclusion and exclusion criteria also were eligible to participate. BAY 94-9027 dosing during the perioperative period was based on preoperative pharmacokinetic measurements and adjusted at the physician’s discretion. Types of procedures and duration of surgeries, BAY 94-9027 consumption on the day of surgery, intraoperative blood loss, surgeon assessment of hemostasis during surgery, and need for blood transfusion were evaluated.
Summary:
17 patients (median [range] age, 37 [13–61] years) underwent 20 major surgeries, including 15 orthopedic surgeries (9 joint replacements [hip, n=1; knee, n=6; ankle, n=2], 2 open synovectomies, 3 arthroscopies, and 1 thigh hematoma evacuation), 3 complex dental extractions, 1 penile prosthesis, and 1 inguinal hernia repair at data cutoff (January 2015). Median (range) surgical duration was 102 (17‒217) minutes, and median (range) total dose used on day of surgery, including preoperative, intraoperative, and postoperative infusions on that day, was 72.4 (43‒136) IU/kg. Median (range) number of FVIII infusions on the day of surgery was 2 (1–3), with 40% of procedures requiring only 1 infusion (preoperative) on that day. Following a median (range) presurgical dose of 52.1 (41–64) IU/kg, the median (range) FVIII level (chromogenic assay; measured in a central laboratory) immediately before the second infusion was 71.6 (44–140) IU/dL; median (range) time between the presurgical and second infusions was 12.3 (3.6–50.0) hours. Hemostasis during surgery was good (13/20; 65%) or excellent (7/20; 35%) for all procedures. Intraoperative blood loss was within expected ranges for all surgeries (median [range], 50 [0–1000] mL), and blood transfusions were required in 4 patients undergoing knee surgeries.
Conclusions:
BAY 94-9027 is efficacious in maintaining hemostasis during major surgeries in adolescents and adults with severe hemophilia A. Excellent or good hemostasis with blood loss as expected was achieved in all surgical procedures, which included major orthopedic surgeries (75% of all procedures), with 40% of patients requiring only a single infusion of BAY 94-9027 on the day of surgery.
Achievement of therapeutic levels of factor VIII activity following gene transfer with valoctocogene roxaparvovec (BMN 270): Long-term efficacy and safety results in patients with severe hemophilia A
Objective:
As a single gene disorder of Factor VIII (FVIII), hemophilia A (HA) is an ideal candidate for gene therapy. We present results from an ongoing Phase 1/2 study of valoctocogene roxaparvovec (BMN 270; AAV5-FVIII-SQ) gene transfer in patients with severe HA.
Methods:
As of 16 April 2018, 13 subjects (6E13 vg/kg, n=7; 4E13 vg/kg, n=6) received a single intravenous dose of valoctocogene roxaparvovec, an AAV5 vector containing a B-domain-deleted FVIII gene. Safety, efficacy, immunogenicity, and other endpoints are being evaluated.
Summary:
FVIII activity is presented as median levels over 4-week intervals. In the 6E13 cohort, FVIII activity plateaued by Week 20 post-valoctocogene roxaparvovec, with median levels between Weeks 20-104 in the non-hemophilic range ([range] 46-122 IU/dL); Week 104 median FVIII activity was 46 IU/dL ([range] 6-145 IU/dL). In the 4E13 cohort, median [range] FVIII activity increased to just below the normal range (NR) at Week 52 [n=6]: 32 [3-59] IU/dL. Prior FVIII prophylaxis subjects had median [interquartile range, IQR] annualized FVIII infusions decline from 139 [122-157] (6E13) and 156 [126-183] (4E13) to 0 [0-0.4] and 0 [0-1] 4 weeks post-infusion through last follow-up; median [IQR] annualized bleeding rates post-infusion were 0 [0-0] in both cohorts (no bleeding episodes in 5 subjects in each cohort). Mild, grade 1, asymptomatic alanine aminotransferase (ALT) increases were reported in six of seven 6E13 and four of six 4E13 subjects; one 4E13 subject had a grade 2 ALT increase. Peak ALT levels ranged from 44-141 U/L (upper limit of normal=43 U/L). All subjects had a normal ALT level at last follow-up and all subjects were off of corticosteroid therapy. No subjects developed inhibitors to FVIII.
Conclusions:
Gene transfer with valoctocogene roxaparvovec in subjects with severe HA resulted in sustained, clinically relevant FVIII activity that reduced self-reported bleeding and exogenous FVIII use 2 years post-infusion in the 6E13 cohort. FVIII activity in the 4E13 cohort was maintained at the upper range of mild HA 1 year post-infusion. Both doses enabled achievement of long-term therapeutic levels of FVIII activity and prevention of hemophilia-related bleeding with a favorable safety profile.
Bypassing agent (BPA) use for the treatment of bleeds in persons with Hemophilia A (PwHA) with inhibitors before and after emicizumab prophylaxis in the HAVEN 1 study
Change in cost and units consumed by people with factor VIII and factor IX deficiency after switching from a standard half-life product to an extended half-life product
Integrated efficacy and safety analysis of Phase 2 and 3 studies with glecaprevir/pibrentasvir in patients with a history of bleeding disorders and chronic hepatitis C virus genotype 1–6-infection
A Multicenter, Retrospective Data Collection Study on the Compassionate Use of a Plasma-Derived Factor X Concentrate to Treat Patients with Hereditary Factor X Deficiency
Objective:
Report results of an open-label international study that collected retrospective data on compassionate use of high-purity plasma-derived FX concentrate (pdFX) in subjects with hereditary factor X (FX) deficiency (FXD).
Methods:
This study included subjects with hereditary FXD (irrespective of severity) who received compassionate use pdFX as routine prophylaxis (RP), on-demand (OD) treatment, short-term prevention, and/or perisurgical hemostatic cover. Dosing was at the investigator’s discretion and tailored to each patient. Data from date of first compassionate use dose until data cutoff (31 December 2015) were collected retrospectively.
Summary:
All 15 enrolled subjects from 12 study centers received ≥1 pdFX dose for compassionate use. Of these, 13 subjects were aged ≥12 years (mean, 22.8 years) and 2 were aged <12 years, 8 (53.3%) were female, 12 (80.0%) were white, 3 (20.0%) were Asian. All subjects had moderate or severe FXD (FX activity [FX:C] <5 IU/dL).Of the 15 patients, 7 received only RP, 7 received only OD, and 1 alternated between OD and RP. The 8 subjects on RP received a total of 1239 RP infusions (mean, 154.9 infusions/subject, range 39–492), with a mean dose/infusion/subject of 32.5 IU/kg. The 2 subjects aged <12 years received larger RP doses than the 6 older subjects (mean doses/infusion/subject of 51.1 vs 26.3 IU/kg).Twelve subjects (8 OD, 4 RP; all aged ≥12 years) reported 88 bleeds (34 minor, 7 major, and 47 not rated); 37 bleeds were menorrhagic, 28 were traumatic, 17 were spontaneous, 4 were other, and 2 had unknown cause. pdFX efficacy was rated as effective for the 79 bleeds (including 1 subdural hematoma) treated with OD pdFX. Mean pdFX dose was 22.2 IU/kg/infusion/subject, with a mean of 9.5 infusions/subject to treat a bleed. More bleeds occurred in the OD than in the RP population.Two subjects underwent 1 dental procedure each, with only 1 presurgical pdFX dose required per patient; a third surgery, a portacath insertion, required 6 infusions to prevent postoperative bleeding. Two successful pregnancies/childbirths were also reported, with no abnormal bleeding complications or efficacy/safety concerns reported.The mean duration of compassionate use was 87.6 weeks for the 15 subjects, with a range of 15–211 weeks (0.3–4.0 years). Over the 1373 infusions administered across 25.2 subject-years, investigators rated overall pdFX efficacy as excellent in 14 (93.3%) subjects and good in 1 (6.7%) subject. No adverse drug reactions, safety concerns, infusion site reactions, tolerability issues, or inhibitor development were reported during pdFX compassionate use.
Conclusions:
The higher bleed rate in OD versus RP use and the treatment duration (up to 4 years) support the efficacy and safety of pdFX demonstrated in prospective clinical studies and its continued use in the treatment of subjects with hereditary FXD.
Staying on TRAQ: Determining transition readiness from pediatric to adult care in adolescents and young adults with hemophilia
Efficacy, safety and pharmacokinetics of once-weekly prophylactic emicizumab (ACE910) in pediatric persons (<12 years) with hemophilia A with inhibitors: interim analysis of single-arm, multicenter, open-label, phase 3 study (HAVEN 2)
Objectives:
Emicizumab, a novel bispecific humanized monoclonal antibody promotes coagulation by bridging FIXa and FX to replace the function of missing activated FVIII, and has potential to address unmet medical needs in pediatric persons with hemophilia A (PwHA) with inhibitors. This study assessed efficacy, safety and pharmacokinetics of once-weekly subcutaneous emicizumab prophylaxis in pediatric PwHA with inhibitors.
Methods:
The study (NCT02795767) enrolled PwHA with inhibitors aged <12 years (or 12–17 years if <40 kg) previously treated with bypassing agents to receive emicizumab prophylaxis for ≥52 weeks. Emicizumab was administered subcutaneously at 3 mg/kg/week for 4 weeks, followed by 1.5 mg/kg/week thereafter. Efficacy objectives included bleed rate, and comparison of the bleed rate on emicizumab prophylaxis vs historical bleed rate obtained from a prospective, non-interventional study (NIS; NCT02476942). The NIS collected detailed, high-quality real- world data on bleeds and safety outcomes from a cohort of pediatric PwHA with inhibitors treated according to local, routine clinical practice. Participants from the NIS could subsequently enter the HAVEN 2 study, which permitted intra-individual comparisons.
Summary:
This interim analysis included 20 PwHA with inhibitors aged 3–12 years (median 8.5); 19 aged <12 years were included in the efficacy analyses. The median observation time was 12.1 weeks (range 7–14). In total, 18/19 (94.7%) participants had zero treated bleeds and 12/19 (63.2%) did not bleed while on study. Overall, 14 bleeds were reported in 7 participants, with none occurring in a joint or muscle. No participants have required up- titration of emicizumab. A substantial reduction in ABR on study vs ABR on prior treatment with bypassing agents (non-interventional study) was observed in 8 participants included in the intra-individual comparison; all 8 participants reported zero bleeds with emicizumab prophylaxis (efficacy period 85–99 days). Emicizumab was well tolerated; most common AEs were mild injection-site reactions (15%) and nasopharyngitis (15%). Three unrelated serious AEs were observed (mouth hemorrhage, appendicitis, catheter site infection). No thromboembolic or thrombotic microangiopathy events were reported. No anti-drug antibodies were detected. Mean trough emicizumab concentrations of >50 μg/mL were achieved after 4 loading doses of 3 mg/kg/week and sustained with maintenance doses of 1.5 mg/kg/week, and were consistent across age groups and body weight.
Conclusion:
Emicizumab prophylaxis was well tolerated and prevented/reduced bleeds in pediatric PwHA with inhibitors. Clinically meaningful reductions in ABR were observed compared with ABR on prior treatment with bypassing agents. The pharmacokinetic profile of emicizumab was similar to that seen in adolescent/adult PwHA with inhibitors. These interim data show the potential for emicizumab to reduce the disease and treatment burden for pediatric PwHA with inhibitors.
Efficacy, safety and pharmacokinetics of emicizumab (ACE910) prophylaxis in persons with hemophilia A with inhibitors: randomized, multicenter, open-label, phase 3 study (HAVEN 1)
Estimating the prevalence of symptomatic, undiagnosed von Willebrand disease: analysis of medical insurance claims data
Physician practice patterns in the US show significant variation in how PK parameters are currently used to personalize care for US hemophilia A patients
An Integrated Safety and Efficacy Analysis of Sofosbuvir-Based Regimens in Patients with Hereditary Bleeding Disorders
Efficacy, safety, and pharmacokinetics of a high-purity plasma-derived factor X (pdFX) concentrate in the prophylaxis of bleeding episodes in children <12 years with moderate to severe congenital factor X deficiency (FXD)
Hereditary factor X (FX) deficiency in women and girls: treatment with a high purity plasma-derived factor X concentrate
Background:
A high-purity plasma-derived FX concentrate (pdFX) has been developed for treatment of hereditary FX deficiency, an autosomal recessive disorder.
Aim:
This post hoc analysis describes the pharmacokinetics, safety, and efficacy of pdFX in 10 women and girls with hereditary FX deficiency.
Methods:
In this open-label study, subjects (10 women/girls, 6 men/boys) aged ≥12 years with moderate or severe FX deficiency (basal plasma FX activity ≤5 IU/dL) were enrolled and received 25 IU/kg pdFX for on-demand treatment of bleeding episodes or preventative use for up to 2 years. All subjects provided informed consent and the protocol was approved by appropriate independent ethics committees.
Results:
Nine women and girls had severe and 1 had moderate FX deficiency, were aged 25.5 (median; range 14–58) y, and received a total of 267 pdFX infusions (178 for on-demand and 89 for preventative treatment). Men and boys (5 severe and 1 moderate FX deficiency) received a total of 159 pdFX infusions (64 on-demand; 95 preventative). The mean number of infusions per subject per month was higher among women and girls (2.48) than males (1.62). The mean pdFX incremental recovery was similar between women/girls and men/boys (2.05 vs 1.91 IU/dL per IU/kg, respectively), as was mean half-life (29.3 and 29.5 h, respectively). Among women and girls, 132 assessable bleeding episodes (61 heavy menstrual bleeding, 47 joint, 15 muscle, and 9 other) were treated with pdFX. Women and girls reported a treatment success rate (ie, subject rating of “excellent” or “good” response to pdFX) of 98%, comparable to the 100% treatment success rate among men and boys. After study completion, 2 subjects received pdFX for hemostatic cover during obstetric delivery. Additional infusion, bleed, and safety data will be presented.
Conclusion:
These results show that, in women and girls with moderate or severe hereditary FX deficiency, who experience reproductive tract and other bleeding events, pdFX was safe and effective. The pharmacokinetic profile of pdFX in women and girls was similar to that of men and boys.
Funding: Bio Products Laboratory
Updated results from a dose-escalation study in adults with severe or moderate-severe hemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy: up to 1.5 years follow-up
An Evaluation of the Switch from Standard Factor VIII Prophylaxis to Prophylaxis with an Extended Half-Life, Pegylated, Full-length Recombinant Factor VIII (BAX 855)
Low Annualized Bleeding Rates in Phase 3 Studies of Recombinant Factor VIII Fc Fusion Protein (rFVIIIFc) in Subjects with Target Joints at Baseline
Efficacy of a Recombinant Factor IX Investigational Product, IB1001 (trenonacog alfa) for Perioperative Management in Hemophilia B Patients
Efficacy of a Recombinant Factor IX Investigational Product, IB1001 (trenonacog alfa) in Previously Treated Patients with Hemophilia B
Recombinant von Willebrand factor in severe von Willebrand disease: a prospective clinical trial
Kids B-LONG: Safety, Efficacy, and Pharmacokinetics of Long-Acting Recombinant Factor IX Fc Fusion Protein (rFIXFc) in Previously Treated Children with Hemophilia B
Pegylated full-length recombinant factor VIII (BAX 855) for prophylaxis in previously treated adolescent and adult patients with severe hemophilia A
Long-term safety and efficacy of recombinant factor VIII Fc (rFVIIIFc) for the treatment of severe hemophilia A: United States subgroup interim analysis of the ASPIRE study
Increase or Maintenance of Physical Activity in Patients Treated with Recombinant Factor IX Fc Fusion Protein (rFIXFc) in the B-LONG and Kids B-LONG Studies
Patients Treated with Recombinant Factor VIII Fc Fusion Protein (rFVIIIFc) Reported Increased or Maintained Physical Activity in the A-LONG and Kids A-LONG Studies
SPINART 3-Year Analyses: Patient- and Joint-Level Changes in Colorado Adult Joint Assessment Scale and Magnetic Resonance Imaging Scores With Bayer’s Sucrose-Formulated Recombinant Factor VIII (rFVIII-FS) in Adolescents and Adults
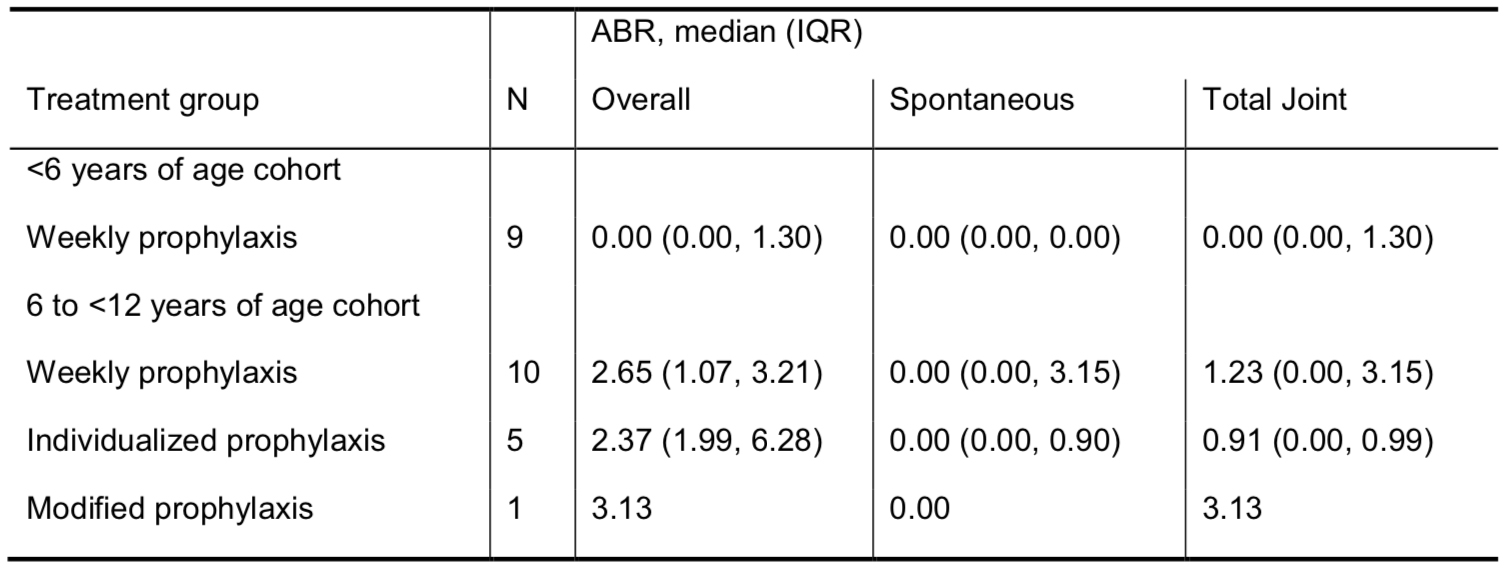
Interim results of the B-YOND study evaluating long-term safety and efficacy of recombinant factor IX Fc (rFIXFc) in children with severe hemophilia B
Objective:
The ongoing rFIXFc extension study, B-YOND (clinicaltrials.gov #NCT01425723) , evaluates the long-term safety and efficacy of rFIXFc for the treatment of severe hemophilia B. Here we report interim safety and efficacy data for children aged <12 yrs enrolled in B- YOND.
Methods:
Upon completing Kids B-LONG, eligible subjects could enroll in one of the 3 prophylactic treatment groups in B-YOND: weekly (20 to 100 IU/kg every 7 days), individualized (100 IU/kg every 8 to 16 days, or twice monthly), or modified (a prophylaxis regimen different from weekly or individualized prophylaxis). Subjects could change treatment groups at any point in the study. The primary endpoint was development of inhibitors. Secondary outcomes included annualized bleeding rate (ABR) and rFIXFc exposure days (EDs).
Summary:
At the time of the interim data cut (17 October 2014), 23 subjects had completed Kids B-LONG; all enrolled in B-YOND (<6 yrs of age cohort, n=9; 6 to <12 yrs of age cohort, n=14). As of the interim data cut, 2 subjects had completed and 21 subjects continued in B-YOND (median time on study: 47.7 weeks). From the start of Kids B-LONG to the B-YOND interim data cut, the median time on rFIXFc was 95.3 weeks, with a median of 94 cumulative rFIXFc EDs. All subjects were on weekly prophylaxis in Kids B-LONG; 5 subjects changed treatment groups at the start of or during B-YOND. In the weekly prophylaxis group, the median (IQR) average weekly prophylactic dose was 64 (52, 66) IU/kg and 63 (59, 64) IU/kg in the <6 yrs and 6 to <12 yrs of age cohorts, respectively. The median (IQR) dosing interval among subjects on individualized prophylaxis was 10 (10, 11) days. As of the interim data cut, no inhibitors were observed, there were no reports of anaphylaxis or serious hypersensitivity reactions associated with rFIXFc, and no thrombotic events. Adverse events were typical of the pediatric hemophilia B population; no subject discontinued the study due to an adverse event. Median ABRs were low in both age cohorts (Table). Overall, 95% of bleeding episodes were controlled with 1 or 2 infusions.
Conclusions:
Interim data in children with severe hemophilia B participating in B-YOND confirm the long-term safety of rFIXFc and the maintenance of a low ABR with extended-interval prophylactic dosing.