Pathway to Cures’ investment in Spark Biomedical is an example of how we foster the development of innovative approaches to healthcare access and unmet medical needs in the blood and bleeding disorders community.
Spark Biomedical’s hemostasis division, FiveLiters, builds on 20 years of research at the Feinstein Institutes for Medical Research in vagus nerve stimulation and hemostasis, coupled with Spark Biomedical’s FDA approved non-invasive neurostimulation device.
Spark Biomedical’s first clinical studies for bleeding disorders are encouraging. When participants used the device for 60 minutes two times per day each day of their period, the result overall was 50% less blood loss and 20% shorter periods. Cramp pain severity was also significantly reduced. The trials, the results of which were presented at NBDF’s Wednesday Webinar May 28th, included 16 participants:
- 8 participants with von Willebrand disease (VWD) type 1 and heavy menstrual bleeding, and
- 8 participants with heavy menstrual bleeding of unknown cause.
At Pathway to Cures we are excited about this because VWD type 1 is one of the most prevalent inheritable bleeding disorders in the world as well as being often undiagnosed and overlooked, limiting access to treatment. Likewise, alternative safe and effective treatments for heavy menstrual bleeding are also needed as the current drug and hormone treatments are not sufficient for many people, and many seeking help for this type of abnormal bleeding are not taken seriously by their health care providers.
To support our community now, Spark Biomedical has launched a wellness brand, OhmBody, with a product designed to improve menstrual symptoms from heavy menstrual bleeding to cramping, mood swings, menstrual brain fog, and gastric upset. Spark Biomedical will continue their FDA clinical trials to develop additional products for bleeding disorders over the next few years.
For more about this approach to managing heavy menstrual bleeding:
- NBDF Wednesday Webinar May 28, 2025 “A New Era of Menstrual Care: The Revolutionary Sciences of Wearable Neurostimulation” discussing the science and clinical studies supporting the use of non-invasive vagus nerve stimulation to manage heavy menstrual bleeding.
- Publications including: a Nature Communications article regarding the mechanism of action of this approach to hemostasis which features the figure below.
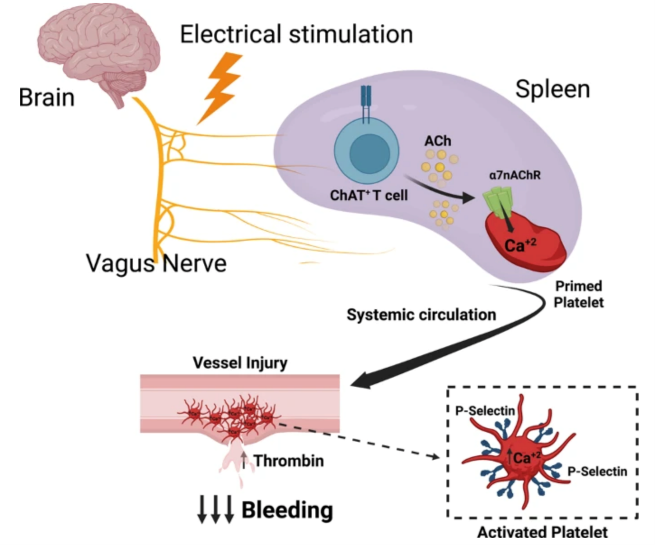
- Recent American Society of Hematology abstracts: Delivering Transcutaneous Auricular Neurostimulation to Regulate Platelet Activity in Healthy Human Subjects (Nov. 2024) and Delivering Transcutaneous Auricular Neurostimulation to Reduce Heavy Menstrual Bleeding in Von Willebrand Disease Patients (Nov. 2024)
- The 2025 Bleeding Disorders Conference (BDC) exhibit hall where a member of the Spark Biomedical team will be happy to talk with you.
- The OhmBody website (ohmbody.com) including the blog Periodically Speaking.
NBDF’s Pathway to Cures is supporting this mission-relevant work to provide access and relief to people who have been dismissed or misdiagnosed when working to understand their bleeding disorder. Spark Biomedical is providing a solution now through a non-invasive, hormone-free wellness product, removing a barrier to care, while continuing development of future FDA-cleared products based on the innovative science of hemostasis through neurostimulation.
Teri Willey, Managing Director & Officer, Pathway to Cures, National Bleeding Disorders Foundation
Tim Brent, Venture Principal, Pathway to Cures, National Bleeding Disorders Foundation





